Claimable Blog
Learn about appeals, denials, and stay up to date on what is happening in the world of healthcare.

On July 1, CVS Caremark began forcing patients to switch from Zepbound to Wegovy – and we quickly took action to help folks fight back by appealing. With many patients protected by step therapy and non-medical switching laws, we were confident in their cases. The majority of these denials should have been overturned easily.
They weren’t.
Our team quickly started noticing an unusual – and troubling – pattern. Appeals were getting denied at a high rate and at unusual speed. Denials were coming back not in the standard hours or days, but in minutes – all following the same script and formula, returned with almost identical responses. Same wording. Same rationale. Same disregard for the patient’s actual medical needs.
Under federal law, every appeal is supposed to get a full, fair, individualized review by a human reviewer. These weren’t reviews. They were copy-paste auto-replies. This falls well outside of what we’ve been used to from insurers, and it raised serious legal concerns.
Seeing the patterns in the data
The appeals process is typically fragmented, with individual patients and providers rarely compiling or comparing notes. Spotting trends is nearly impossible. But by handling hundreds of appeals specifically for CVS’s Zepbound forced-switch patients, Claimable had a unique vantage point. We saw systemic, policy-wide denials unfolding in real time. These weren’t a few isolated cases; we were seeing a consistent, repeated pattern of patients being denied their legal rights.
We immediately began supporting second-level appeals and escalation to independent review, including a detailed opinion from our Senior Legal Advisor, D. Brian Hufford, Esq., of The Hufford Law Firm PLLC, to help patients fight for the coverage they deserved. More appeals began to succeed – but not nearly enough.
Our success rate doubled after escalating cases with stronger legal arguments, but it remained below our usual benchmarks. That wasn’t good enough. We knew something was deeply wrong. So even while individual appeals were starting to work, it was clear that this broader pattern of systemic denials raised bigger legal questions – questions that went beyond what the appeals process alone can fix.
So with Brian, we began investigating additional options.
The CVS Caremark Zepbound lawsuit and your right to a full, fair, individualized review
Working closely with patients we’d supported through their appeals, Brian took the evidence to Berger Montague, a firm that specializes in healthcare class action litigation.
On September 3, 2025, they filed a class action lawsuit against CVS Caremark on behalf of patients in ERISA-governed employer-sponsored health plans whose coverage for Zepbound was denied and whose appeals were rejected based on medical necessity.
The lawsuit alleges that CVS Caremark wrongfully denied coverage by issuing denials that appeared to rely on templated language, despite patients meeting the plans’ criteria for medical necessity. Filed under ERISA, the suit alleges that CVS Caremark:
- Breached its fiduciary duties by prioritizing financial gain over medical appropriateness or plan obligations;
- Engaged in prohibited transactions by entering formulary agreements that benefit its own bottom line;
- Violated the terms of employer health plans by denying coverage for an FDA-approved, medically necessary treatment – while steering patients toward non-equivalent or off-label alternatives; and
- Ignored federal claims procedure standards by failing to provide timely, transparent, and individualized appeal reviews.
The complaint asks the court to issue injunctive relief, requiring CVS to change its policies going forward. It also seeks other appropriate equitable relief if those remedies are found insufficient to fully address the harm to patients.
Advocacy doesn’t end with the appeal
Since July 1st, we’ve helped hundreds of patients file appeals for Zepbound denials. That’s only a tiny slice of the hundreds of thousands of patients affected. But it’s enough to spot the trend and push for accountability.
To be clear: Claimable isn’t a party to this suit. The relief it seeks isn’t on our behalf. But for us, being a patient-first company means taking a root cause approach to solving problems whenever possible. In this case, it meant going beyond the appeals process we operate within and connecting patients to legal options they might not otherwise access.
We built Claimable to make appealing easier and more successful. But just as importantly, we built it to expose what’s really happening behind the scenes. Denials don’t happen in isolation, and neither can our response.
That’s why we’re proud to support a broader movement for change, alongside legal teams, advocacy organizations, and policy leaders. Appeals are one piece. Litigation is another. Legislative reform is critical too. The only way to deter unjust denials is to challenge them—again and again—until insurers and pharmacy benefit managers face real consequences for saying no without cause.
What’s next for Zepbound appeals
Legal action takes time, and we’ll be watching closely as this case makes its way through the courts. But while the system may be slow, we’re not slowing down. We will continue helping patients appeal these Zepbound forced switches – and we’ll keep evolving our strategies as new evidence and appeal precedents emerge.
We hope this lawsuit sends a clear message: insurer misconduct that puts patients at risk will not go unnoticed or unchallenged.
Our job isn’t just to make paperwork easier and arguments stronger. It’s to fight back when something feels wrong. To listen to patients. To advocate. To act.
And we won’t stop until everyone gets the care they need and coverage they deserve.

Asthma isn't often thought of as a critical condition; but for many, access to medication for it is life-saving.
In 2024, Cole Schmidtknecht's insurance denied his steroid inhaler. Shortly after, he suffered cardiac arrest induced by a severe asthma attack, and passed away following an ICU stay – just eleven days after he had to choose between paying his rent and picking up his prescription.
Since then, his parents Bil and Shanon Schmidtknecht have worked tirelessly to share Cole's story and advocate for the PBM reform that could have saved his life. In this letter, they share the real, human cost of asthma denials – and why giving people a path to coverage is so incredibly critical.

Dear Claimable Team,
We're reaching out with deep gratitude and shared purpose – as parents, advocates, and people who know all too well what it means when access to asthma medication is delayed or denied.
Asthma is not a mild or temporary inconvenience – it is a chronic, life-threatening disease that requires consistent, uninterrupted access to prescribed medications. When an insurer denies coverage for a prescribed asthma treatment – whether it's a maintenance inhaler, rescue inhaler, or biologic – it is not simply a paperwork issue. It is a decision that can disrupt care, cause physical harm, and in the most tragic cases, lead to death.
Our son, Cole Schmidtknecht, died following a sudden asthma attack during one of the happiest times in his life. He had been fighting through the obstacles put in place by a broken healthcare system – including delays, denials, and unaffordable pricing. The denial of coverage for a medically necessary asthma medication can cost someone their life.
We live with that reality every day.
Appealing a denial is not just a bureaucratic step – it is a lifeline. When insurers reconsider their decision based on additional clinical context or urgency, they have the power to correct a dangerous mistake and prevent suffering. It's not only the right thing to do – it's a matter of life and death.
We want to extend our heartfelt thanks to each of you at Claimable for taking the initiative to bring asthma denial cases into your platform. Creating a simple, accessible path for patients and families to challenge harmful decisions is a powerful act of compassion – and a concrete step toward justice and accountability in healthcare.
Most importantly, we urge everyone – patients, caregivers, providers, and even insurers-to fight back when access to care is denied. Always appeal. Always ask questions. Always push for what is right.
Because every delay, every rejection, every barrier can cost someone more than just time – it can cost them their life.
Thank you for being part of the solution, and for honoring lives like Cole's through the work you do.
With gratitude,
Bil and Shanon Schmidtknecht
Patient Advocates
Justice for Cole and All Others

In 2024, the American Academy of Pediatrics published a Clinical Report on PANS. Despite being explicitly labeled "not a clinical guideline", this report has begun to be used by pediatrics providers, leading to failures in diagnosing and treating PANS/PANDAS.
Worse still, this report has been systematically misused by insurance providers to deny care for these patients – claiming that well-studied, evidence-backed treatment has no medical basis and thus, these patients and families have no claim to Insurance coverage for it. These denials are inappropriate and harms these young patients.
At Claimable, our role in fighting denials goes beyond generating appeals; it's about holding insurers accountable to fair, medically sound, and evidence-based practices. In this letter, we've collaborated with leading PANS/PANDAS organizations to bring light to what the science actually supports – and get these patients the treatment they deserve.
JOINT STATEMENT FROM FOUR NATIONAL PANS/PANDAS NON-PROFITS AND CLAIMABLE, A COMPANY SUPPORTING AFFECTED FAMILIES
Four leading PANS/PANDAS organizations, ASPIRE, NWPPN, PANDAS Network and the Look. Foundation, together with Claimable, a company providing advocacy and support for families navigating PANS/PANDAS, are raising serious concerns about the American Academy of Pediatrics’ (AAP) 2024 Clinical Report on PANS/PANDAS.
The AAP Report is not a clinical guideline—yet some pediatricians are citing it to block diagnosis and treatment, and by insurers to justify denials of critical therapies, including IVIG. This misuse is deeply concerning and has real-world consequences.
The report:
- Omits key studies supporting the use of IVIG and steroids
- Conflicts with peer-reviewed clinical guidelines from institutions like Stanford, Columbia, and the NIMH
- Lacks transparency regarding authorship and expert input
- Advises against important diagnostic tools like strep testing
- As a result, children are being misdiagnosed, undertreated, or denied care altogether—leading to psychiatric crises, medical complications, and devastating impacts on families.
We respectfully urge the following actions:
- The AAP should retract and revise the report to address its omissions and clarify its intended use
- Pediatricians should rely on the established, peer-reviewed clinical guidelines
- (JCAP) for diagnosing and treating PANS/PANDAS
- Insurers should stop using the report to deny care and revisit all IVIG denials based on it
This is about more than policy—it's about protecting children from preventable suffering. We stand united in calling for an evidence-based, compassionate, and transparent approach to care.
DATE: July 25, 2025
RE: Rebuttal to the American Academy of Pediatrics Clinical Report on PANS (12/16/24)
TO:
Dr. Susan J. Kressly, M.D., FAAP, President, Board of Dir. American Academy of Pediatrics: 345 Park Boulevard Itasca, IL 60143
Dr. Pamela K. Shaw, M.D., FAAP, President-Elect, Board of Dir. American Academy of Pediatrics: 345 Park Boulevard, Itasca, IL 60143
Dr. Moira A. Szilagyi, M.D., Ph.D., Past Pres.-Elect, Board of Dir. American Academy of Pediatrics: 345 Park Boulevard, Itasca, IL 60143
Dr. Brian P . Sanders, M.D., FAAP, Secretary/Treas., Board of Dir. American Academy of Pediatrics: 345 Park Boulevard, Itasca, IL 60143
Mark D. Del Monte, J.D. CEO/Exec.VP , Board of Dir. American Academy of Pediatrics: 345 Park Boulevard, Itasca, IL 60143
Robert F . Kennedy Jr, Sec. of Health & Human Serv: Hubert H. Humphrey Building, 200 Independence Avenue, S.W.
CC:
Cory Harris, President & CEO, Wellmark: 1331 Grand Avenue, Des Moines, IA 50309
David Cordani, Chairman & CEO, The Cigna Group: 900 Coage Grove Road, Bloomfield, CT 06002
David Joyner, President, CVS Caremark: 1 CVS Drive, Woonsocket, RI 02895
Gail Boudreaux, President & CEO, Elevance Health: 220 Virginia Avenue, Indianapolis, IN 46204
Joseph Zubretsky, President & CEO, Molina Healthcare: 200 Oceangate, Suite 100, Long Beach, CA 90802
Kimberly Keck, President & CEO, Blue Cross Blue Shield Assoc.: 200 E. Randolph Street, Chicago, IL 60601
Sarah London, CEO, Centene Corporation: 7700 Forsyth Boulevard, St. Louis, MO 63105
Stephen Helmsley, CEO, UnitedHealth Group: 9900 Bren Road East, Minnetonka, MN 55343
Dear Drs. Kressly, Shaw, Szilagyi, and Sanders, Mr. Del Monte, and Secretary Kennedy:
We are compelled to respond to the American Academy of Pediatrics' (“ AAP”) Pediatric Acute-Onset Neuropsychiatric Syndrome (PANS): Clinical Report (“Report”) published on December 16, 2024, which is now being misused by pediatric providers to diagnose and treat PANDAS/PANS, as well as by insurers to justify the denial of IVIG coverage.The AAP itself explicitly states that this document is not a clinical guideline and should not be used to dictate treatment decisions:
"Because they are limited by the present level of evidence on the topic, the findings are presented as a report rather than a clinical practice guideline."
Despite this disclaimer, pediatric providers are misapplying the report by disregarding established clinical guidelines, leading to failures in properly diagnosing and treating PANDAS/PANS. At the same time, insurers are misusing the report as definitive evidence against IVIG, ignoring its limitations, lack of transparency, and omied research. This misrepresentation threatens access to critical treatment and delays necessary medical care..
OUR IMMEDIATE REQUEST
Given the serious flaws in the AAP Report and the inappropriate ways in which it is being used, we call for the following immediate actions.:
- The AAP must retract this report until its flaws are corrected and its intended use is clarified. Its lack of transparency, omitted research, and misrepresentation of IVIG make it unreliable and misleading.
- The imperative of medical rule-out in psychiatric diagnoses: Under the DSM-5 guidelines, mental health practitioners and physicians must rule out any underlying medical cause before assigning a psychiatric diagnosis. However, much of this report relies heavily on psychiatric labeling. Failing to perform an “etiological medical rule-out” not only risks medical negligence but can also deny patients access to accurate, potentially life-saving diagnoses and treatments.
- Insurers must stop using this report to deny care. It is not a clinical guideline, and its misuse harms families and obstructs physician-led treatment.
- All IVIG denials based on this report must be reconsidered immediately in light of strong medical evidence supporting its use for moderate-severe PANS/PANDAS.
CONSEQUENCES OF A FAILURE TO ACT
The failure to act allows pediatricians and insurers to continue leveraging an incomplete document at the expense of patient care, forcing children into needless suffering and irreversible harm. Without treatment, these children may experience a panoply of consequences, including:
- Severe neuropsychiatric decline, leaving them unable to speak, eat, or attend school
- Malnutrition and medical starvation, requiring hospitalization and feeding tubes
- Increase in autoimmune biomarkers demonstrated in 54.2% of children in a 2024 cohort study by Ma et al
- Psychiatric crises, leading to emergency interventions and long-term disability
- Permanent cognitive and developmental regression, forcing families into financial hardship
- Fatalities - The POND brainbank was established in 2022 with the goal of understanding the pathogenesis and mechanisms associated with PANS/PANDAS. The 11 specimens within the brain bank were donated by the families of their deceased children.
When pediatric providers rely on a non-guideline document to guide diagnosis and treatment, it undermines evidence-based care and delays appropriate interventions, worsening outcomes and increasing long-term costs. Likewise, denying IVIG does not reduce costs—it escalates them, shifting the burden to emergency hospitalizations, feeding interventions, and more expensive treatments like plasmapheresis. Both examples fail to recognize established diagnostic and treatment guidelines published by subject experts having worked in the field of PANDAS/PANS for 30 years.
Why the AAP Report Fails as Pediatric Clinical Guidance and Justification for IVIG Denial
- It Is Not a Clinical Guideline.
The AAP explicitly states that this report does not provide official treatment recommendations. It lacks consensus-based standards and does not establish clear directives for insurers to follow. This may represent misuse of clinical reports in medical practice.
- Impedes Access to Testing and Diagnosis.
The 2024 AAP Report on PANS/PANDAS restricts key diagnostic tools, advising against routine streptococcus testing (e.g., throat cultures, rapid antigen tests) unless classic symptoms like pharyngitis are present, and discouraging brain imaging (e.g., MRI) unless focal neurological signs suggest alternative diagnoses such as autoimmune encephalitis. The imposed limitations risk delaying or preventing accurate diagnosis, making it harder for families to access appropriate testing, specialist care, and insurance coverage—ultimately obstructing timely intervention for children with acute-onset neuropsychiatric symptoms while driving up longer term direct and indirect costs to families, employers and insurers.
- Lack of Transparency and Expert Input
The AAP report does not disclose its authors or the specialists consulted, raising serious credibility concerns. There is no indication that experts in pediatric neuroimmunology, infectious disease, or immunology—fields essential to understanding PANS/PANDAS as well as IVIG’s role—were involved.
- The AAP did not consult any of the major multidisciplinary university clinics that research and treat PANS/PANDAS. These institutions represent thousands of cases of collective experience, yet their input was neither sought nor included.
"By failing to adequately disclose potential conflicts of interest, the AAP violated transparency standards, calling the report’s validity into question. According to International Committee of Medical Journal Editors (ICMJE) standards:
"Authors should disclose relationships and activities that readers could perceive to have influenced, or that give the appearance of potentially influencing, their work. This includes, but is not limited to, relationships with for-profit and not-for-profit third parties whose interested may be affected by the content of the manuscript."
- Omits Key Studies and Is Already OutdatedThe review period for the AAP report ended in 2023, meaning it fails to incorporate newer research—including Melamed et al. (2024)— which demonstrated statistically significant improvements in OCD-related symptoms following IVIG treatment. Additionally, the omission of studies on the use of steroids to shorten the duration of flares.Even within its stated review period, the report omitted clinical guidelines and studies that support the use of steroids and IVIG, including: 1. Swedo, Cooperstock, Frankovich, Thienemann et al. (2017): Consensus Guidelines explicitly include IVIG as a recommended treatment for severe cases. These expert-driven guidelines remain a valid and authoritative clinical framework.2. Pavone (2020), Hajjari (2022), and Eremija (2023): All demonstrated IVIG’s effectiveness for severe or persistent cases.3. Melamed et al. (2021): A multi-site, open-label study of 21 patients over six monthsb showed measurable improvements in psychological function with IVIG. Results were statistically significant.4. Brown K, Farmer C, Farhadian B, Hernandez J, Thienemann M, Frankovich J. (2017): Corticosteroids may be a helpful treatment intervention in patients with new-onset and relapsing/remitting PANS and PANDAS, hastening symptom improvement or resolution. When corticosteroids are given earlier in a disease flare, symptoms improve more quickly and patients achieve clinical remission sooner. Additionally, the AAP report selectively dismisses positive IVIG studies due to small sample sizes while accepting weak or inconclusive evidence against IVIG without applying the same scrutiny, introducing bias into its conclusions.
Further, standard prescribing and coverage policies routinely approve off-label pediatric treatments (Carmack et al., 2020; Allen et al., 2018; Shah et al., 2007) and therapies for small patient populations using similar evidence standards. PANS/PANDAS is widely accepted to be a rare disease, and thus large multi-arm randomized and double-blinded studies are methodologically impractical.
A 2025 randomized, placebo-controlled Phase III study (NCT04508530) of PANZYGA® in PANS patients demonstrated a clinically meaningful reduction in CY-BOCS scores (31. 1% improvement vs. 12. 1% in placebo), though the p-value narrowly missed statistical significance (p = 0.072).
However, the secondary endpoint—the Clinical Global Impression scale (CGI-I)—achieved statistical significance (p = 0.017), validating PANZYGA®'s real-world clinical benefit across multiple domains of functioning. These findings directly contradict the AAP Report’s implication that evidence for IVIG is weak or unreliable.
Denials Based on This Report Contradicts Cost-Saving Measures
Blocking access to IVIG is not only harmful—it is financially irresponsible. Calaprice et al. (2023) found that unrestricted access to care for PANS results in more symptom-free days, significantly reducing the need for costly hospitalizations, psychiatric admissions, emergency interventions, and lost wages for caregiving parents.
When IVIG is denied, families face greater financial and medical burdens, including:
- Severe psychiatric hospitalizations
- Feeding tube interventions due to OCD-driven food refusal
- Plasmapheresis, a far more expensive treatment that could have been avoided with IVIG
Families who are denied access based on the AAP Report have been forced to pay out of pocket, which serves to further widen the disparities associated outcomes along socioeconomic lines. Access problems caused by sequential denials may only be ameliorated with access to legal counsel, which again is generally not widely available to many families.
PEDIATRIC PROVIDERS ARE FAILING TO PROPERLY DIAGNOSE AND TREAT PANDAS/PANS — AND INSURERS ARE MISUSING THE AAP REPORT TO JUSTIFY DENIALS.
Many pediatric providers are citing the AAP Report to justify treating PANS/PANDAS solely as a psychiatric condition—directly contradicting peer-reviewed, evidence-based guidelines developed by the PANS Research Consortium. These guidelines were published in JCAP in 2017 and 2019, authored by leading experts from institutions including Stanford, Columbia, and the NIMH. Yet due to the AAP’s institutional weight, its flawed report is often prioritized over these more specialized clinical standards.
This widespread clinical misapplication is not occurring in isolation–insurers are seizing on it to justify treatment denials and further restrict access to care.
A review of over 100 denied PANS/PANDAS-related cases shows an accelerating paern since early 2025: insurers are increasingly invoking the 2024 AAP Report in ways that distort its intent. Although the report explicitly states it is not a clinical guideline, it is being treated as one—used to justify denials and restrict access to care, often without appropriate clinical justification or specialty input.
For example, Wellmark justified a denial by stating:
"A recent AAP review article-position published recently indicates essentially nothing has changed regarding the utilization of IVIG in the treatment of presumed PANS-PANDAS."
Here, Wellmark conflates the AAP Report and the outdated AAP Red Book, using both to assert a lack of evidence while disregarding more current, supportive data.
Premera Blue Cross similarly cited the AAP to dismiss IVIG, asserting:
"In 2024, the American Academy of Pediatrics (APP) published a clinical report in which their expert panel concluded... there are no well-designed trails that provide evidence-based guidance on treatment for PANS' symptoms..."
Premera’s framing misrepresents the evidence base, and the denial falsely implies that psychiatric and antibiotic care alone is sufficient—despite acknowledging that PANS is likely a valid diagnosis.
These examples demonstrate a dangerous pattern: insurers are using a non-guideline report to undermine medical judgment, deny treatment access, and sidestep robust clinical evidence.
CONCLUSION
The AAP report is not a clinical guideline and should not be used to dismiss diagnosis or treatment of PANS/PANDAS by pediatric providers, nor to justify IVIG denials. Coverage decisions must be based on clinical needs and strong medical evidence supporting IVIG for PANS/PANDAS.
We urge the following immediate actions:
- Pediatricians must stop using the flawed AAP report in place of well-established, evidence-based clinical guidelines that are essential for the proper diagnosis and treatment of PANS/PANDAS.
- The AAP should retract the report until its flaws are addressed and its intended use clarified.
- Insurers must stop using the report for utilization management to deny care.
- Insurers must reconsider all IVIG denials based on this report.
- Employer fiduciaries of self-funded plans must ensure the AAP report is not used to restrict care.
- State Departments of Insurance should sanction insurers for misusing unsound evidence in unsound coverage determinations.
The continued misuse of this non-guideline report to dismiss diagnosis and deny treatment is an unacceptable violation of the principles of non-maleficence (do no harm) and beneficence (act in the patient’s best interest). Denying IVIG based on this flawed report is medically unsound, financially reckless, and ethically indefensible—contradicting clinical guidelines, increasing long-term costs, and undermining clinician authority.
Sincerely,
Gabriella True, President, ASPIRE
Warris Bokhari, MD, CEO & Co-Founder, Claimable
Jennifer M. Vitelli, MBA, Executive Director, Look. Foundation
Sarah Lemley MPA; HA, Executive Director, Northwest PANDAS/PANS Network
Diana Pohlman, Executive Director, PANDAS Network
COMPARISON OF AAP REPORT CLAIM VS. LITERATURE EVIDENCE
REFERENCES
CLINICAL RESEARCH STUDIES
Calaprice-Whitty D, Tang A, Tona J. Factors associated with symptom persistence in PANS: Part I-Access to care. J Child Adolesc Psychopharmacol. 2023;33(9):356-364. doi:10. 1089/cap.2023.0022. Epub 2023 Oct 30. PMID: 37902790.
Kulumani Mahadevan LS, Murphy M, Selenica M, Latimer E, Harris BT . Clinicopathologic characteristics of PANDAS in a young adult: a case report. Dev Neurosci. 2023;45(6):335-341. doi:10. 1159/000534061. Epub 2023 Sep 12. PMID: 37699369; PMCID: PMC10753865.
Pavone P , Falsaperla R, Cacciaguerra G, et al. PANS/PANDAS: clinical experience in IVIG treatment and state of the art in rehabilitation approaches. NeuroSci. 2020;1:75–84. doi:10.3390/neurosci1020007.
Melamed I, Kobayashi RH, O’Connor M, et al. Evaluation of intravenous immunoglobulin in pediatric acute-onset neuropsychiatric syndrome. J Child Adolesc Psychopharmacol. 2021;31(2):118-128. doi:10. 1089/cap.2020.0100.
Hajjari P , Oldmark MH, Fernell E, et al. Pediatric acute-onset neuropsychiatric syndrome (PANS) and intravenous immunoglobulin (IVIG): comprehensive open-label trial in ten children. BMC Psychiatry. 2022;22(1):535. doi:10. 1186/s12888-022-04181-x. PMID: 35933358; PMCID: PMC9357317.
Eremija J, Patel S, Rice S, Daines M. Intravenous immunoglobulin treatment improves multiple neuropsychiatric outcomes in patients with pediatric acute-onset neuropsychiatric syndrome. Front Pediatr. 2023;11:1229150. doi:10.3389/fped.2023. 1229150. PMID: 37908968; PMCID: PMC10613689.
Melamed I, Rahman S, Pein H, et al. IVIG response in pediatric acute-onset neuropsychiatric syndrome correlates with reduction in pro-inflammatory monocytes and neuropsychiatric measures. Front Immunol. 2024;15:1383973. doi:10.3389/fimmu.2024. 1383973.
Vreeland A, et al. Postinfectious inflammation, autoimmunity, and obsessive-compulsive disorder: Sydenham chorea, pediatric autoimmune neuropsychiatric disorder associated with streptococcal infection, and pediatric acute-onset neuropsychiatric disorder. Dev Neurosci. 2023;45(6):361-374. doi:10. 1159/000534261.
Carmack M, Hwang T , Bourgeois FT . Pediatric Drug Policies Supporting Safe And Effective Use Of Therapeutics In Children: A Systematic Analysis. Health A (Millwood). 2020 Oct;39(10):1799-1805. doi: 10. 1377/hlthaff.2020.00198. PMID: 33017255.
Allen HC, Garbe MC, Lees J, Aziz N, Chaaban H, Miller JL, Johnson P , DeLeon S. O-Label Medication use in Children, More Common than We Think: A Systematic Review of the Literature. J Okla State Med Assoc. 2018 Oct;111(8):776-783. PMID: 31379392; PMCID: PMC6677268.
Zheng J, Frankovich J, McKenna ES, et al. Association of Pediatric Acute-Onset Neuropsychiatric Syndrome With Microstructural Differences in Brain Regions Detected via Diffusion-Weighted Magnetic Resonance Imaging. JAMA Network Open. 2020;3(5):e204063. doi:10. 1001/jamanetworkopen.2020.4063.
Johnson M, Ehlers S, Fernell E, et al. Anti-Inflammatory, Antibacterial and Immunomodulatory Treatment in Children With Symptoms Corresponding to the Research Condition PANS (Pediatric Acute-Onset Neuropsychiatric Syndrome): A Systematic Review. PloS One. 2021;16(7):e0253844.
Trifiletti R, Lachman HM, Manusama O, et al. Identification of Ultra-Rare Genetic Variants in Pediatric Acute Onset Neuropsychiatric Syndrome (PANS) by Exome and Whole Genome Sequencing. Scientific Reports. 2022;12(1):11106
Xu J, Frankovich J, Liu RJ, et al. Elevated Antibody Binding to Striatal Cholinergic Interneurons in Patients With Pediatric Acute-Onset Neuropsychiatric Syndrome. Brain, Behavior, and Immunity.
Shah SS, Hall M, Goodman DM, et al. Off-label drug use in hospitalized children [published correction appears in Arch Pediatr Adolesc Med. 2007 Jul;161(7):655]. Arch Pediatr Adolesc Med. 2007;161(3):282-290. doi:10. 1001/archpedi. 161.3.282.
Michael Daines, MD (PI). A Superiority Phase 3 Study to Compare the Eect of Panzyga Versus Placebo in Patients with Paediatric Acute-Onset Neuropsychiatric Syndrome, protocol NGAM-13.
Perlmutter SJ, Leitman SF , Garvey MA, Hamburger S, Feldman E, Leonard HL, et al. Therapeutic plasma exchange and intravenous immunoglobulin for obsessive-compulsive disorder and tic disorders in childhood. Lancet. 1999; 354(9185):1153–8. https:/ /doi.org/10. 1016/S0140-6736(98)12297-3.
Kovacevic M, Grant P , Swedo S. Use of Intravenous Immunoglobulin in the Treatment of Twelve Youths with Pediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal Infections. J Child Adol Psychopharm 2015; 25(1): 65-69. DOI: 10. 1089/cap.2014.0067
Pavone P , Falsaperla R, Nicita F , et al. Pediatric Autoimmune Neuropsychiatric Disorder Associated with Streptococcal Infection (PANDAS): Clinical Manifestation, IVIG Treatment Outcomes, Results from a Cohort of Italian Patients. Neuropsychiatry 2018; 8(3): 854-860. DOI:10.4172/Neuropsychiatry. 1000412
LaRusso M, Gallego-Pérez DF , Abadía-Barrero CE. Untimely care: How the modern logics of coverage and medicine compromise children's health and development. Soc Sci Med. 2023 Feb;319:114962. doi:
10. 1016/j.socscimed.2022. 114962. Epub 2022 Apr 6. PMID: 35584978.
Tang AW, Appel HJ, Bennett SC, et al. Treatment barriers in PANS/PANDAS: Observations from eleven health care provider families. Fam Syst Health. 2021;39(3):477-487. doi:10. 1037/fsh0000602
Calaprice-Whiy D, Tang A, Tona J. Factors Associated with Symptom Persistence in PANS: Part I-Access to Care. J Child Adolesc Psychopharmacol. 2023;33(9):356-364
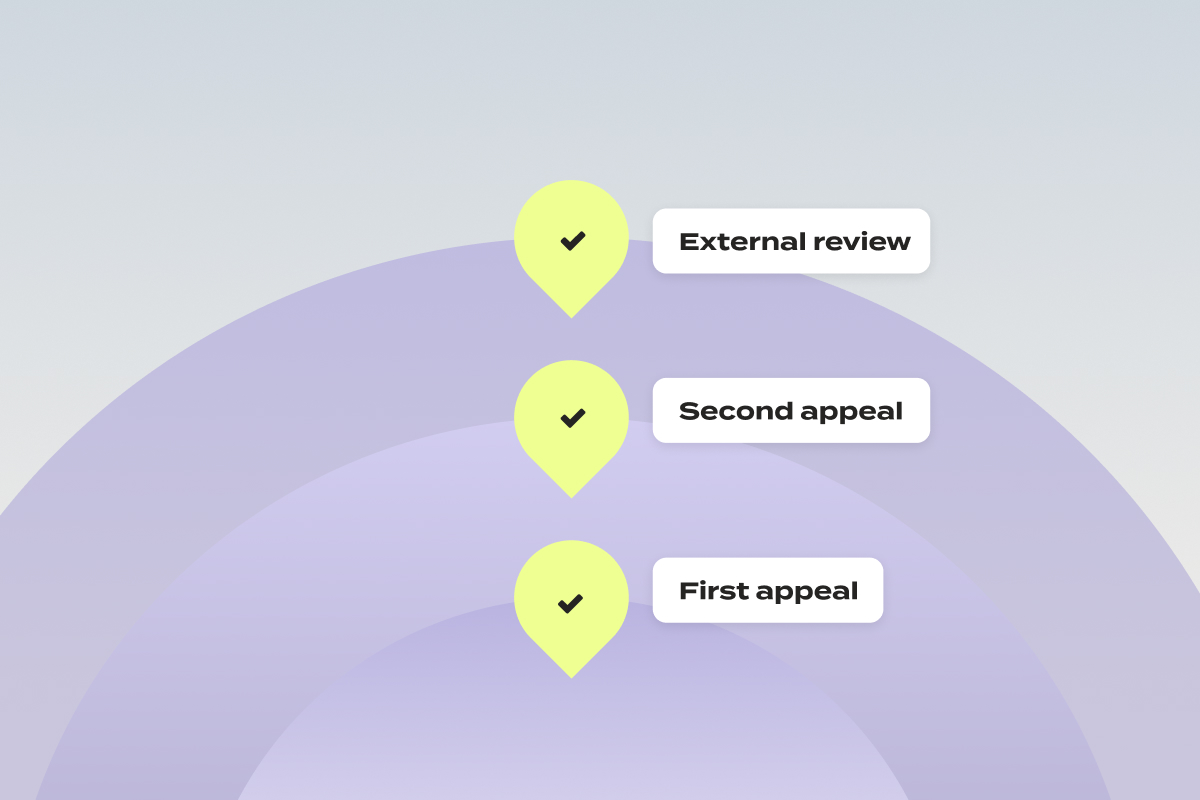
At Claimable, we’re dedicated to empowering patients to fight for the care that they deserve. That’s why, as of today, we’re officially supporting free second-level appeals for CVS Caremark Zepbound forced-switch denials — so you can be confident your case receives a full, fair, and individualized review.
First, What’s A Second-Level Appeal?
When you submit an appeal as a patient and it’s denied, it isn’t the end. You have the right to request a second-level appeal, which often involves review by a different department or an independent third party. Put simply? It’s another chance to have your case heard, and have your denial overturned.
Why We’re Escalating to Second-Level Appeals
Over the past weeks, we’ve had an influx of patients file appeals for Zepbound coverage with CVS Caremark. Unfortunately, we’ve begun to see responses to those appeals come back as form-letter denials – ignoring not only your unique health history, doctor’s notes, and plan details, but also state and federal laws. Federal rules are clear: every appeal must receive an individualized review. The copy paste answers we’re seeing? They’re not that.
In order to make sure that each of these appeals get the full and fair review they’re entitled to, it’s time to escalate it to the next level:
- Internal vs. External Reviews
- Internal appeal: Your denial is reviewed by CVS Caremark or your insurer. Yes, the same company that issued the denial – though federal law requires that the review be conducted by someone who wasn’t involved in the original decision.
- External appeal: Also called an external review or, in some states, an Independent Medical Review (IMR). This is conducted by a licensed medical professional or organization not affiliated with your insurance provider, using objective, evidence-based criteria. External decisions are binding on the insurer, but you may still have the right to pursue legal action if the outcome is unfavorable.
For forced-switch denials of Zepbound, we pursue the external review – most likely to secure the coverage you need — free of charge.
What to Expect If Your First Zepbound Appeal Is Denied
- You Report the Denial
Once you get the notification that your appeal has been denied, simply log into your Claimable account to report the outcome – or email us at support@getclaimable.com - You Consent To Escalation
We’ll confirm you’d like to continue to escalate to a second-level appeal. At this point, you’ll upload your appeal denial letter. - We Build Your Strongest Case
Once you consent, we’ll generate your second-level appeal package. This includes a newly drafted appeal letter with an expert legal opinion and your prior appeal materials. - You Review & Submit
We’ll notify you when your second-level appeal is ready for review.You’ll confirm where to send (fax/mail) your appeal, then click “Submit” once everything looks right. - We Send – Expedited
Claimable will fax and mail your appeal to both CVS and the independent reviewer.
Because all Zepbound forced-switch appeals involve ongoing treatment for a serious condition, your case will be marked urgent – requesting an expedited external review. This means that in most cases, the reviewer must issue a decision within 72 hours, though some may take longer.- You Get Notified
You’ll be notified of the outcome via portal message, email, or phone, as well as receiving a mailed copy of the decision.
If it’s approved? The decision is binding – and CVS must cover your Zepbound.
Key Questions – Answered
What is a second-level appeal?
It’s your right to request another review when your first appeal is denied—either internally by the insurer or externally by an independent reviewer. We choose the path most likely to win for you.
Why is Claimable offering free 2nd-level appeals?
With these early Zepbound appeals, we’ve seen a clear pattern of “cookie-cutter” denials that violate the full-fair-and-individualized-review requirement. To level the playing field, we’re offering these escalations free of charge to demand unbiased consideration.
Keep in mind, this only applies if you submitted your first-level appeal through Claimable. If you've appealed a different way and think you might need a second-level appeal, feel free to email us for help navigating the process.
What’s included in my Claimable second-level appeal?
A freshly drafted appeal letter with expert legal commentary, your first appeal materials, and previous denial letters—packaged to maximize your chance of success.
Is there any cost to second level appeals?
Nope – this service is completely free for CVS Caremark Zepbound forced-switch cases. Claimable’s appeal strategy is customized for each insurer and medication—and in these cases, supporting a second-level appeal is part of our core approach to winning.
What do I need to submit a second-level appeal?
You’ll need your appeal denial letter to confirm the instructions for submitting a second-level appeal, including where to send it and whether any forms are required.
If you’ve received your claim file and designated record set since initially submitting, we recommend uploading them when prompted (after you review your second-level appeal draft).
How do I track my appeal’s status?
If you haven’t heard back within 72 hours, call the number on your insurance card. Otherwise, we’ll notify you as soon as the reviewer issues a decision.
Do I have to redo the appeal questionnaire?
Never. All your first-level answers and uploads carry over automatically.
We know the appeals process can feel confusing and overwhelming. That’s why we built Claimable — to guide you every step of the way. With our new second-level appeal support, you can rest assured that we’ll fight tirelessly to get you the coverage you deserve.
Questions? We’re here for you. Reach out to support anytime.


The past few years have brought major progress in how we prevent and manage migraine. CGRP-targeting medications like Aimovig, Emgality, Vyepti, Nurtec ODT, and Qulipta represent a shift in both the science and strategy of treating this condition.
They're often better tolerated and more effective than older medications, and they allow for more personalized care, whether that means a daily pill, a monthly injection, or a quarterly infusion.
But while the science has advanced, insurance coverage hasn’t kept up. Patients are still being denied access to these medications due to outdated policies, arbitrary formulary changes, and one-size-fits-all coverage rules. At Claimable, we’re working with patients, providers, and advocacy partners to challenge these barriers—and win.
If you’re facing a denied claim for a migraine medication, read on to understand the tactics insurance companies take to limit access to these treatments – and what you can do to get covered.
Understanding the migraine treatment landscape
As a migraine sufferer, I know that migraines are more than a bad headache. It’s a disabling neurological disease that affects over 40 million people in the U.S. and is a leading cause of missed work and reduced quality of life.
CGRP (calcitonin gene-related peptide) medications have transformed our ability to treat and prevent migraine. Unlike older medications developed for other conditions (like epilepsy or depression), CGRP drugs are designed specifically for migraine. They target the biological mechanisms believed to drive attacks, offering relief with fewer side effects.
Why migraine coverage is so challenging
Even though these medications are FDA-approved and supported by professional guidelines, insurance plans often:
- Require you to fail older, less effective drugs first
- Only cover one CGRP drug despite clinical differences
- Force patients to switch medications mid-year due to rebate deals
- Approve treatment only temporarily, requiring re-authorization every few months
- Use vague or bureaucratic reasons to deny access altogether
This system doesn’t reflect the complexity of migraine or the individual needs of patients. It reflects cost-saving tactics that delay care.
Common migraine insurance denial reasons – and how we fight them
Step Therapy Requirements
What it is: Insurance insists you try and fail older medications (like triptans, topiramate or amitriptyline) before approving CGRP drugs.
Why it’s wrong: These older medications often come with tough side effects and weren’t designed for migraine. The American Headache Society supports CGRP drugs as a first-line option.
How we fight it: We submit detailed appeals outlining your treatment history, side effects from older meds, and professional guidelines that justify bypassing step therapy.
Formulary exclusions
What it is: Only one CGRP medication is covered. All others are denied.
Why it’s wrong: CGRP drugs aren’t interchangeable. A daily pill may work better for one person than a monthly injection. Side effects and effectiveness vary.
How we fight it: We explain the medical rationale for your chosen medication, using provider notes and evidence that shows why it’s not just a preference—it’s a necessity.
Mid-Year medication switching
What it is: Your plan changes coverage mid-year due to PBM rebate deals, forcing you to switch medications from a medication you’re stable on to what they prefer.
Why it’s wrong: Migraine treatment relies on consistency. Switching meds can cause rebound attacks and destabilize your care.
How we fight it: We focus on treatment stability and cite ERISA protections (if applicable) to challenge the fairness of mid-year changes.
Short-term or conditional approvals
What it is: You get approved for 30 or 60 days at a time, with constant re-authorization requirements.
Why it’s wrong: Migraine is chronic. Short-term approvals create anxiety, disrupt care, and burden providers.
How we fight it: We argue for long-term approval based on your condition and medication response, using both legal framing and clinical support.
"Not medically necessary" determinations
What it is: Your insurer denies a medication without a clear reason, claiming it’s not necessary.
Why it’s wrong: This ignores your provider’s judgment and contradicts clinical guidelines.
How we fight it: We present peer-reviewed studies, your provider’s rationale, and documentation showing how the treatment improves your quality of life.
Administrative or documentation barriers
What it is: Missing forms, technicalities, or unclear instructions result in denials.
Why it’s wrong: The insurer is making medical decisions by burying you in paperwork. Most people give up and concede to insurance demands. It’s generally understood that patients who accept non-medical switches have adverse side effects and poorer outcomes.
How we fight it: We ensure everything is submitted cleanly and correctly, with language that anticipates common administrative objections.
How Claimable builds strong migraine appeals
As a patient, you have the right to challenge these denied claims. Insurers are required to comply with state and federal laws – which often require their denial rationale to be based on medical necessity, FDA standards, and other clinical, legal, and policy standards.
That means a strong appeal should include clinical evidence, legal standards, and policy compliance to hold insurance accountable to deliver your care and coverage. At Claimable, we use a multi-layered approach:
- Personal narrative: We capture your history, prior treatments, and how migraine affects your life. In particular this could be how you’re not able to enjoy your hobbies or do basic things like drive safely at night. We hear of patients becoming socially isolated.
- Clinical evidence: We include the latest guidelines, studies, and medication-specific data. This includes the guidance from the American Headache Society, and other peer-reviewed studies and randomized controlled trials proving these medications are right for you.
- Legal leverage: We reference plan terms, medical necessity requirements, and ERISA protections. This helps reinforce your rights to have a full and fair review of your care. An insurance doctor who has never met you shouldn’t get to make the call.
- Collaborative advocacy: Your primary care doctor or neurologist can refer you for an appeal if they have a denial, or you can simply add their letter of medical necessity to your appeal to increase its strength.
Why this work matters – unlocking migraine coverage
The medications now available can change lives. But they only work if you can access and stay on them. No one should be forced to suffer just because their insurer hasn’t updated its playbook.
At Claimable, we don’t just file paperwork. We build appeals that reflect who you are, what you’ve been through, and why your treatment plan matters. If your migraine medication has been denied, disrupted, or downgraded, we’ll help you fight back—with precision, evidence, and persistence. Your journey matters to us.
Facing an insurance denial for Aimovig, Emgality, Nurtec or other migraine medication?
We’re here to help. Let’s make the system work for you—not against you.
Get started on your migraine appeal today.

The first responses to Zepbound appeals for the CVS Caremark forced switch have started to come in. Claimable COO Alicia Graham breaks down what's in these new denial letters, and what that means next.
If you've gotten one of these letters, know that we're going to keep fighting it. We don't believe this rationale is based on medical necessity, nor that it will hold up to a secondary review. We're actively escalating these appeals, because we believe that you will win.
This thing is getting more ridiculous by the day. As we start to see responses come back to CVS Caremark, I wanted to share what we've been seeing – because frankly, a lot of what they’ve said so far is vague, misleading and even straight up false. I don't believe these denials are in compliance with the plan policy, applicable laws, or clinical standards of care at all, and we're going to break down why that Is.
This is my take on exactly what’s in these denial letters, what it actually means, and what you can do if you got one. I know how confusing this is, and I hope this can help anyone navigating it.
The one size fits all “coverage request was not approved” denial letter
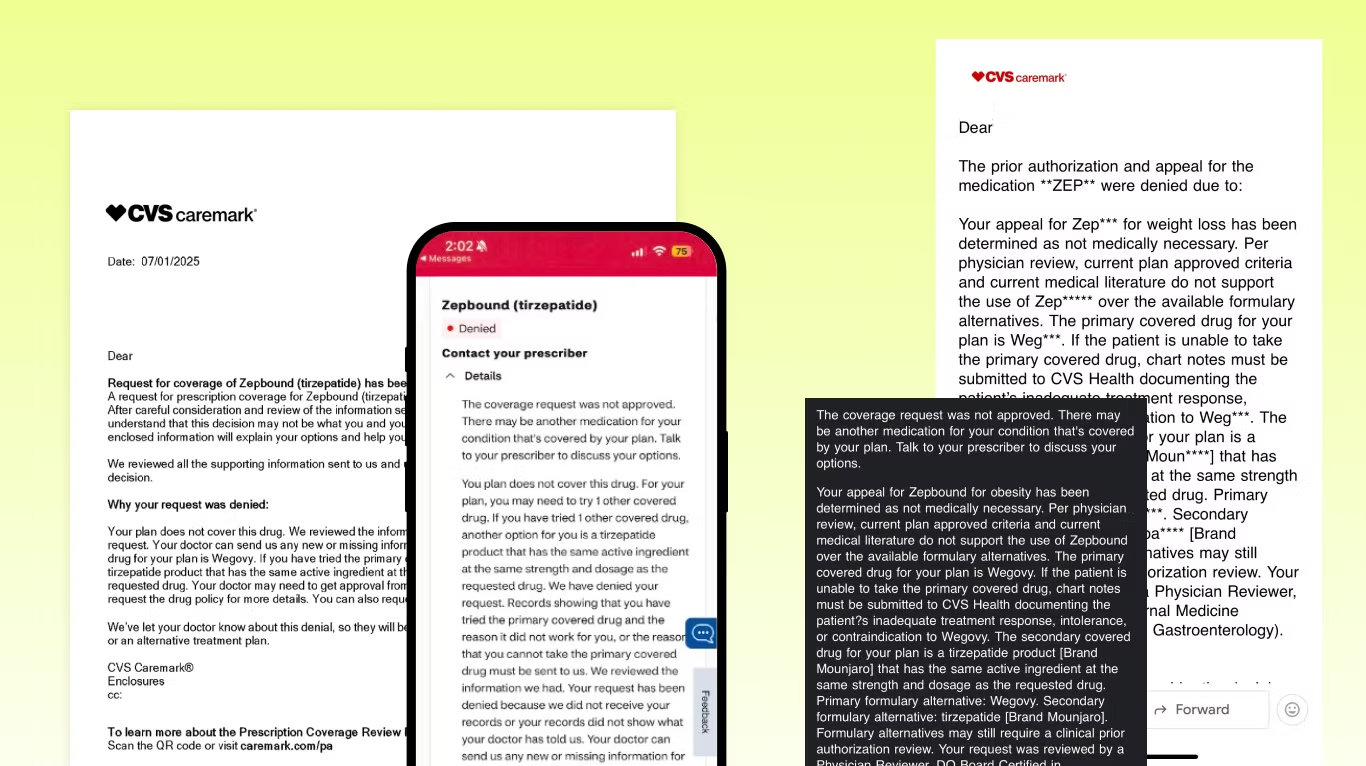
First of all, this is the language we’re seeing in almost all of these letters – just copy-pasted for everyone. By law, each appeal needs to be reviewed by a qualified human performing a full and fair review of specific case facts… so straight off the bat, it doesn’t look like they’re doing that.
“Your appeal for Zepbound has been determined as not medically necessary”
They say that current medical literature doesn’t support the use of Zepbound over the available formulary alternatives. Except it does (here's the head-to-head study we’ve been including in our appeals). They conveniently don’t include the studies they use to make their decision, which they’re supposed to do… so we have no idea why they ignored it and what they are using instead.
Plus, they’re saying Zepbound isn’t medically necessary, but Wegovy and Mounjaro are?! I don’t understand how that argument could ever stand up in a court. Wegovy is indicated for the same treatment with same coverage criteria, and Mounjaro... isn’t even indicated for weight loss.
“The primary covered drug for your plan is Wegovy”
“If the patient is unable to take the primary covered drug, chart notes must be submitted to CVS Health”. What they’re saying here is if you fail Wegovy, you could get back on Zepbound. This is step therapy, but in lots of states there are laws against this. If you live in New York, for example, they cannot require you to have previously failed Wegovy – but we’re seeing them do it anyway.
If you live in New York and got this, you should definitely fight it.
“The secondary covered drug for your plan is a tirzepatide product [Brand Mounjaro]”
This is a curveball – not what we expected to see. Yes, Mounjaro is a tirzepatide like Zepbound, but per the FDA it is only approved for type 2 diabetes.
If you and your doctor agree on you taking Mounjaro off-label, great! That’s completely up to you and what’s best for your care. But for your insurer to try to force you onto an off-label indication? Unethical, clinically absurd, and probably illegal.
But even more importantly this smells like a trap to me.
The trap: “Formulary alternatives may still require a clinical prior authorization”
They’re saying that Mounjaro may still require a PA. We’ve seen this before, so I want patients and providers to clearly understand the risk of going down this path.
The PA form states you do not qualify for Mounjaro unless you have type 2 diabetes, so the minute they ask for a PA your Mounjaro coverage will be gone. Even if they approve you without a PA today, at any time they can come back ask for it which will kick you off.
This has happened to several patients we’ve worked with who took Mounjaro for months and then were told they don’t qualify. They can force you off at any time because the criteria for Mounjaro is different.
The trap within the trap: The 180 day appeal window
I think a big part of them trying to lure you into doing Wegovy or Mounjaro is that by law, you only have 180 days to appeal your Zepbound denial. Hypothetically they could cover Mounjaro for those 180 days, and then on day 181 when that window expires and you’ve lost your right to fight for Zepbound, revoke your approval for Mounjaro – leaving you with nothing.
Even if you want to try Wegovy or Mounjaro, I would keep going on your Zepbound appeal at the same time. If you win a Zepbound appeal, they can’t just take it away.
Robocalls, app messages, and other nonsense
Legally, they have to send you a formal letter with details, full appeal rights, and more. But instead, they’re sending these mini-messages ahead of time and telling you to wait for the letter.
Why? Because not having all the info makes your response weaker and slows things down. Make sure you request your full claim file, formal denial letter, drug policy and all other decision documents. You’re legally entitled to them.
The bottom line: You don’t have to let them win
The TLDR; these denials don’t pass my smell test for compliance with the plan policy, applicable laws, or clinical standards of care. I don’t think these will hold up in external review, courts, or with regulators. So I say keep fighting.
If you got one of these, first request all the documents. Then, escalate it. In your denial letter there will be a number/address for the external reviewer to send it to. **You have the right for an external review and I think that if you do you will win.
At my company this is exactly what we’re doing. We’re creating secondary appeals that address every single piece of BS in these letters and escalating them to reviewers and regulators. Because PBMs and insurers are watching this – and the last thing I want is to show them that pulling stuff like this is ok. It’s not, and we’re going to fight it. And win.
– Alicia
PS – if you only have the “a change is coming on July 1” letter
I’ve seen a lot of people try to appeal using these letters – this will not work. This letter is not a denial of coverage for Zepbound. All this tells you that they will deny it if coverage is requested after July 1, but you can’t appeal based on this.
If you’ve only gotten this letter and want to try to stay on Zepbound, you need to get denied first. That means:
- Your provider needs to submit a new prior authorization request for Zepbound (form here)
- It’s best to do this with a LOMN attached (template here, Lilly has one also)
- You wait until that prior authorization gets denied
- Then you can appeal
Let's get you covered.
